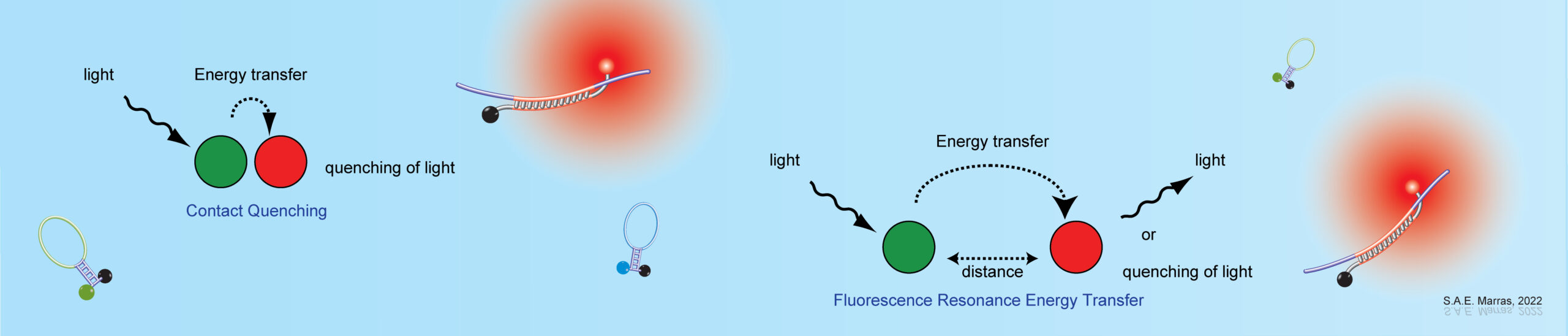
Modes of energy transfer between fluorophores and quenchers
Fluorescence Resonance Energy Transfer
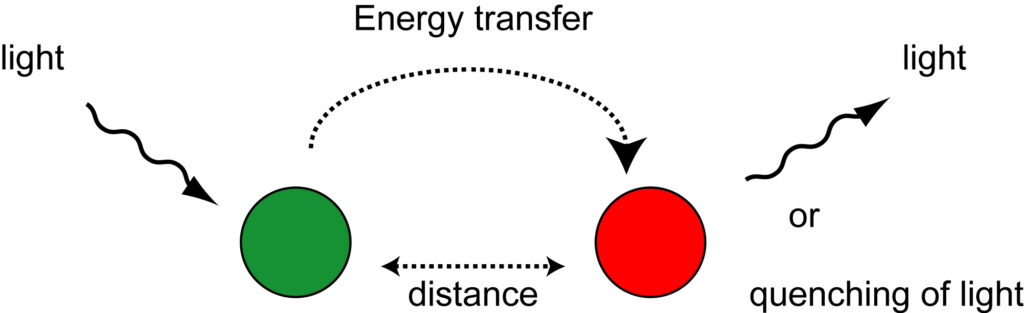
One mechanism of energy transfer between two molecules is fluorescence resonance energy transfer (FRET) or Förster type energy transfer. During this process, a photon from an energetically excited fluorophore, the ‘donor’, raises the energy state of an electron in another molecule, the ‘acceptor’, to higher vibrational levels of the excited singlet state. As a result, the energy level of the donor fluorophore returns to the ground state, without emitting its own fluorescence. This mechanism is dependent on the dipole orientations of both molecules and is limited by the distance between the two molecules. Typical effective distances between the donor and acceptor molecules are in the 10 to 100 Å range. This is roughly the distance between three to thirty nucleotides located in the double helix of a DNA molecule. Another requirement is that the fluorescence emission spectrum of the donor molecule must overlap the absorption spectrum of the acceptor molecule. The acceptor molecule can be another fluorophore or a non-fluorescent molecule. If the acceptor molecule is a fluorophore, the transferred energy can be emitted as fluorescence, characteristic for that fluorophore. If the acceptor molecule is not fluorescent, the absorbed energy is lost as heat, and no fluorescent light is emitted from the complex. Examples of hybridization probes that utilize FRET for energy transfer between two molecules are adjacent probes and 5′-nuclease probes, which are described under hybridization probes.
Contact quenching
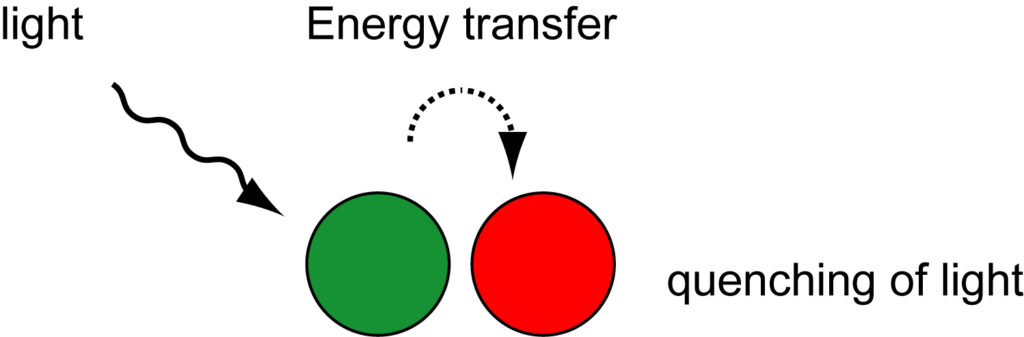
Quenching of a fluorophore can also occur as a result of the formation of a non-fluorescent complex between a fluorophore and another fluorophore or non-fluorescent molecule. This mechanism is known as ‘contact quenching,’ ‘static quenching,’ or ‘ground-state complex formation.’ In contact quenching, two molecules interact by proton-coupled electron transfer through the formation of hydrogen bonds. In aqueous solutions, electrostatic, steric and hydrophobic forces control the formation of hydrogen bonds. When this complex absorbs energy from light, the excited state immediately returns to the ground state without emission of a photon and the molecules do not emit fluorescent light. A characteristic of contact quenching is a change in the absorption spectra of the two molecules when they form a complex. In contrast, in the FRET mechanism, the absorption spectra of the molecules do not change. Among the hybridization probes that use this mechanism of energy transfer are molecular beacon probes and strand-displacement probes, which are described under hybridization probes.
Collisional quenching
Another mechanism that decreases the fluorescence intensity of a fluorophore is called ‘collisional quenching’ or ‘dynamic quenching.’ Collisional quenching occurs when a fluorophore that is raised to its excited state, is deactivated upon contact with another molecule in the same solution. Upon contact, the fluorophore returns to the ground state without emission of fluorescence light. The extent of quenching depends on the nature of the fluorophore, its structure, and the manner of its interaction with the other molecule. Examples of molecules that can act as collisional quenchers are oxygen, halogens, and amines. For example, ethidium bromide becomes fluorescent when it is intercalated within double stranded DNA, since it is protected from oxygen quenching, this compared to ethidium bromide that is free in solution and quenched by oxygen molecules. In 1993, Higuchi and his coworkers described the first real-time quantitative PCR assay, in which they followed the amplification of a DNA sequence in the presence of ethidium bromide.